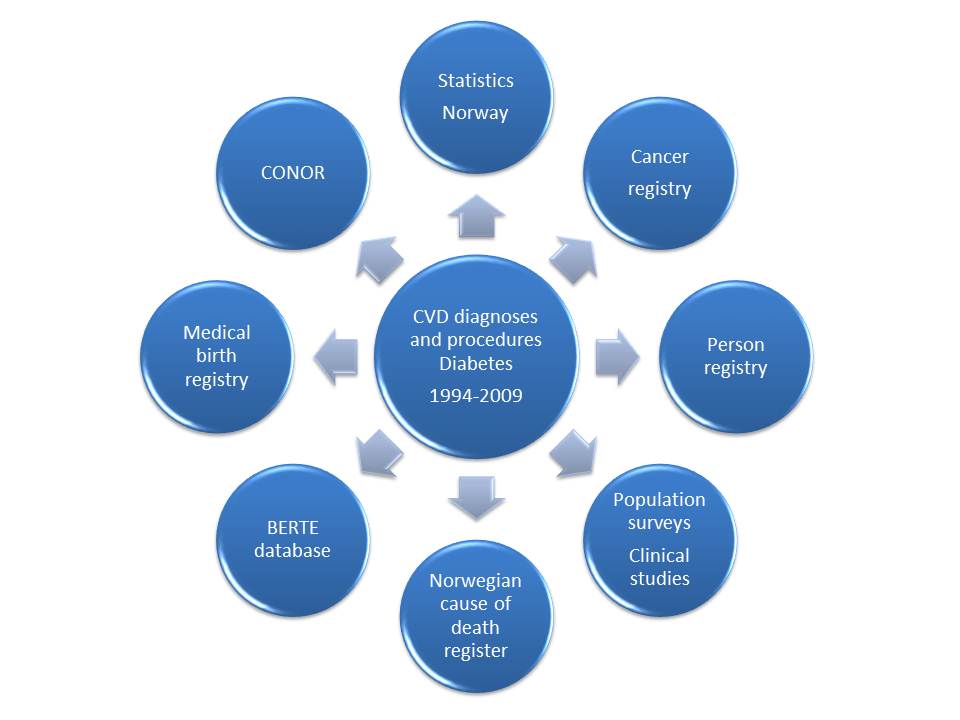
CVD diagnoses and procedures, and Diabetes Mellitus (DM)
The core data set in CVDNOR includes information about all hospitalizations during which a CVD diagnosis or procedure, or DM diagnosis, is mentioned. This information has been retrieved from the Patient Administrative Systems at all Norwegian hospitals during 1994-2009, with the aid of the FS-system (Forskning i sykehus), developed by Tomislav Dimoski at the Norwegian Knowledge Centre for the Health Services. For hospitalizations containing at least one eligible diagnosis or procedure code, all other diagnosis and procedure codes for that stay were extracted.
FS data include a unique ID-number for each patient and a unique code for each hospitalization. In addtion, age at hospitalization, gender, municipality, time and dates of hospitalization and discharge (including transfers between wards and departments within the hospital), hospital, department and ward codes, main and secondary diagnoses (up to 20), medical procedure codes (up to 30) performed while in the hospital, and information about type of hospitalization (acute or elective) and type of referral. Hospitalizations less than 24 hours apart are merged and considered as one.
Other data sources (see below) are linked to the hospital data on CVD and DM, in specific projects. No single project includes information from all these sources.
• Cause of Death Register
For all patients hospitalized with a CVD or DM diagnosis and who later died, information about time, date and underlying cause of death is retrieved from the Cause of Death Registry. In addition, information on individuals who died from CVD or DM but had no previous hospitalizations for CVD or DM is also obtained.
• Statistics Norway, Population Register
Sociodemographic information includes marital status, personal and family income, country of birth, own education, education of spouse, occupation and municipality.
• COhort of NORway (CONOR)
CONOR is a collection of data from several regional health surveys in which standardized information on health behaviours and health status has been obtained. A whole blood sample / DNA is stored.
• Other health surveys and clinical trials
CVDNOR data may also be used as endpoints to several other health surveys including the Norwegian Counties Studies and the Hordaland Health Studies. In addition, CVDNOR data are used as endpoints for the randomized clinical trials such as, The Norwegian Vitamin Trial (NORVIT) and Western Norway B Vitamin Intervention Trial (WENBIT).
• The Medical Birth Registry of Norway
Data from this registry are used in a CVDNOR substudy on congenital heart defects. The overall purpose of this study is to describe the epidemiology of congenital heart defects, examine national time trends and age, period and cohort effects in the prevalence of congenital heart defects in Norway.
• The Norwegian Cancer Registry
Data from this registry are used in a CVDNOR substudy on CVD in cancer survivors; The CVDCancer study. The purpose of this study is to examine the occurence of CVD in cancer survivors, in the general population, and in CONOR participants.